Organic Chemistry Section
Department of Inorganic and Organic Chemistry
Research
Research Lines
People
Links
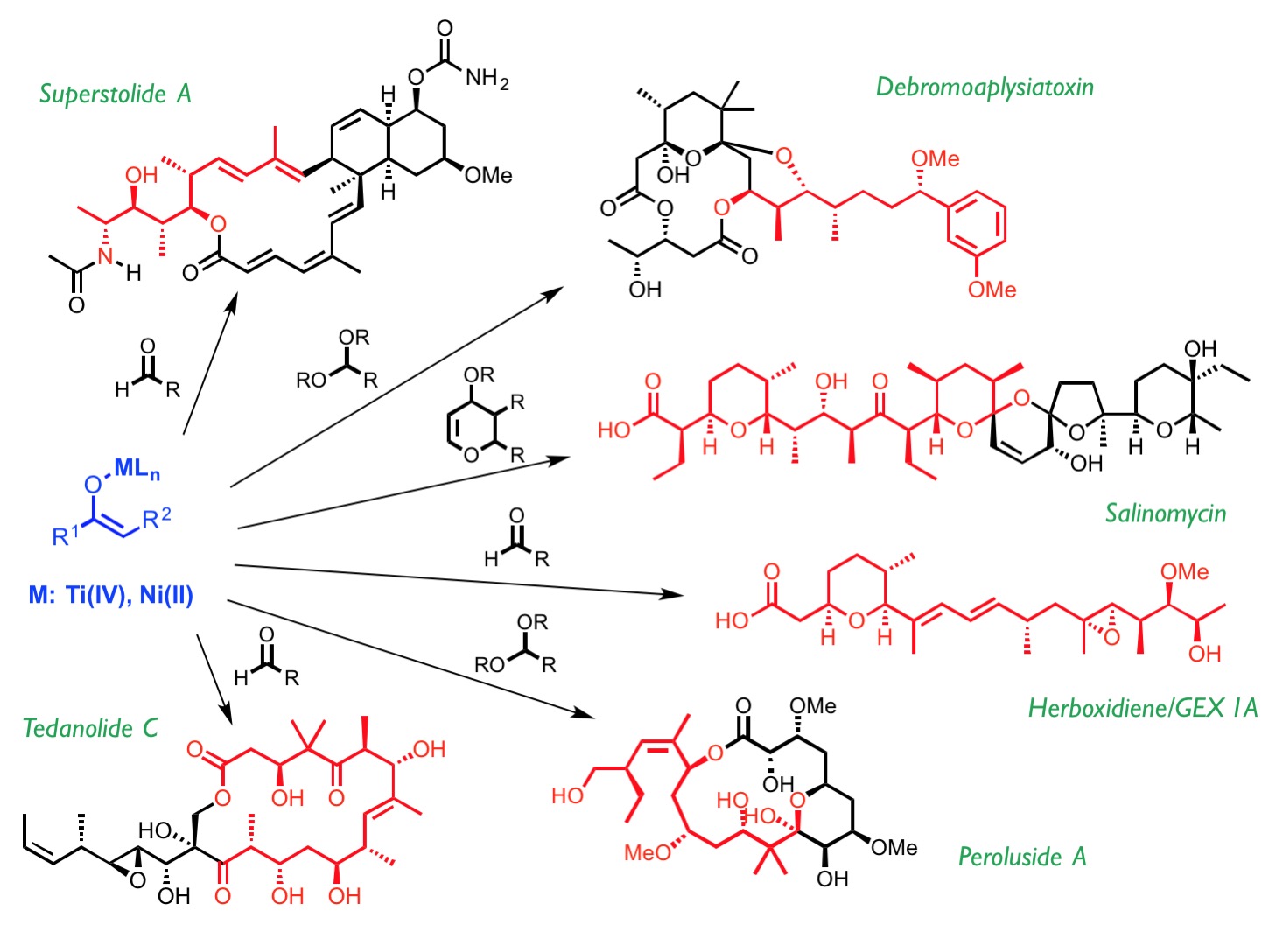
Our research activity revolves around the development of wide-ranging, efficient stereoselective processes based on the reactivity of metal enolates and their application to the synthesis of biologically active compounds.
We are currently involved in the study of direct and stereoselective additions of N-acyl thioimides to a wide range of electrophiles catalyzed by nickel(II) complexes. Our attention is especially focused on SN1-like transformations that may proceed through open transition states in which the high reactivity of carbenium and oxocarbenium intermediates generated in situ can be used for the asymmetric construction of carbon–carbon bonds in alkylation, aldol, Michael or Mannich reactions.
Likewise, the discovery of the biradical character of titanium(IV) enolates has enabled us to use these enolates for the stereoselective construction of carbon-carbon and carbon-oxygen bonds through radical mechanisms.
As stated before, we aim to apply all of these stereoselective transformations to the synthesis of natural products, which in turn is a source of inspiration and a test bed for developing new, simple, effective asymmetric synthesis methods.